NIH Provides Over $3M for Novel Nanotechnology to Detect Cancer in Biofluids
The project seeks to establish a microscopic platform as a seconds-fast tool for cancer detection
The National Cancer Institute, part of the National Institutes of Health, has provided over $3 million in support of a University of California, Davis, project to develop nanotechnology capable of diagnosing cancer from drops of saliva and other biofluid samples.
J. Sebastian Gomez-Diaz, seated, and Randy Carney pose in Gomez-Diaz's Applied Micro/Nano Electromagnetics Research Laboratory. (Photo courtesy of Randy Carney)
J. Sebastian Gomez-Diaz and Randy Carney collaborate across disciplines to develop and test the new diagnostic device. Gomez-Diaz is an associate professor and Chancellor's Fellow in the Department of Electrical and Computer Engineering, and Carney is an associate professor in the Department of Biomedical Engineering.
"The goal is to put forward a new portable neural network platform that [can] analyze several biofluids — like saliva or blood — inside a small and compact device [that will] combine and then utilize the portion of the infrared spectrum of those biofluids for cancer detection," Gomez-Diaz said.
The team is designing the compact device, more than 100 times smaller than a penny, as a cost-effective tool for infrared absorption, or IR, spectroscopy.
IR spectroscopy uses sensors to differentiate molecules based on how much light energy each one picks up. The idea is that each molecule has a distinctive absorptive fingerprint. When visualized, these infrared fingerprints look like an audio file: scraggly lines with sharp peaks and short valleys.
"Infrared absorption spectroscopy is used in basic chemistry labs up to clinical diagnosis, but it's an expensive technique that requires a bulky and difficult-to-use apparatus and yields complicated data for expert interpreters," Carney said.
That's where the neural network comes in. By joining this identification method with machine learning capabilities, the team aims to accelerate and simplify the process.
"We're going to identify the minimum number of those soundwave-like peaks that we need to make diagnostic decisions with machine learning, and our preliminary data indicate that it's very few," Carney said. "Each of the miniature sensors can be tuned to one of those peaks, with the sensor output fed directly to the machine learning algorithm."
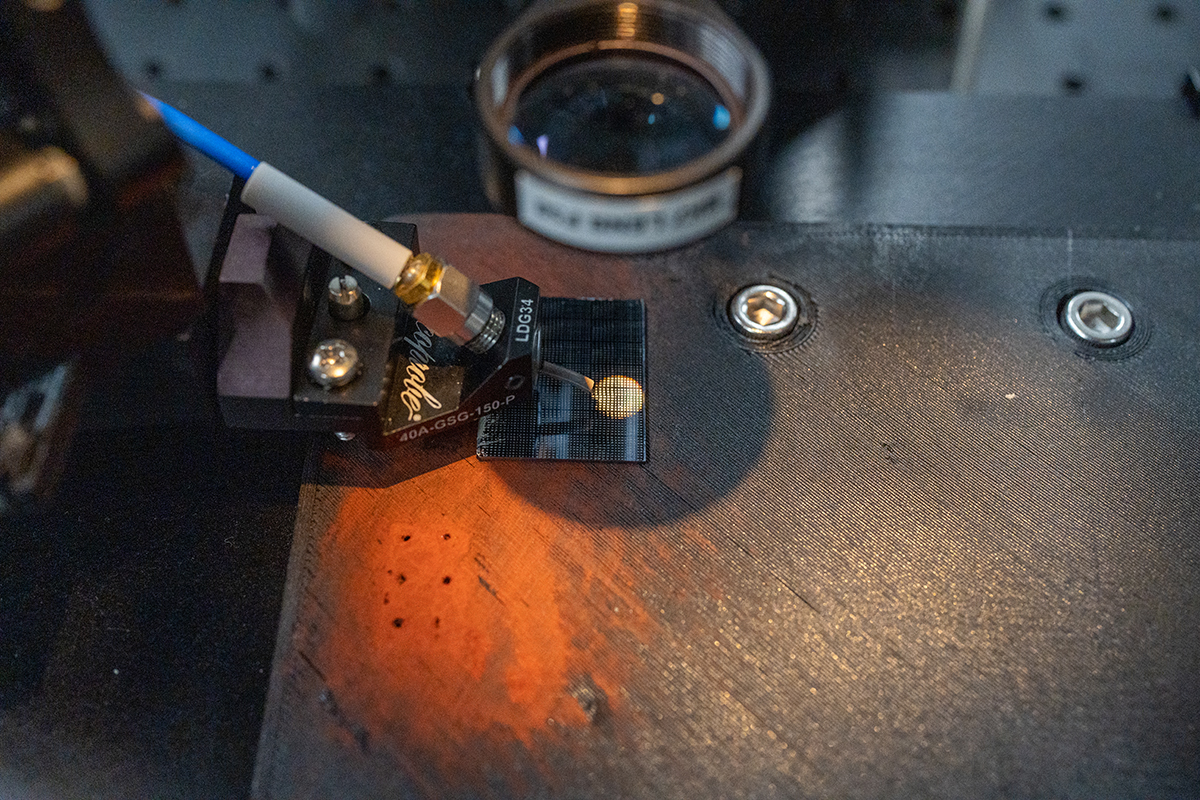
The team uses a bespoke device to train the chip's neural network with infrared light. (Steven Trinh/UC Davis)
The team will focus on using their device for head and neck cancer detection at this stage, with assistance from Andrew Birkeland, a head and neck oncologist and assistant professor at UC Davis Health. Birkeland is also part of UC Davis Health's Comprehensive Cancer Center, which provided the seed funding for early data collection on this project.
What sets this technology apart from past efforts is the neural network's ability to notice patterns in the full spectrum of a saliva sample. Other projects have used significant energy to isolate a single molecule or metabolite for diagnostic purposes.
"By training the neural network with samples with and without cancer, we can identify patterns that allow us to implement the technology and to come into a performance of over 90 percent to distinguish if a sample has cancer or not," Gomez-Diaz said.
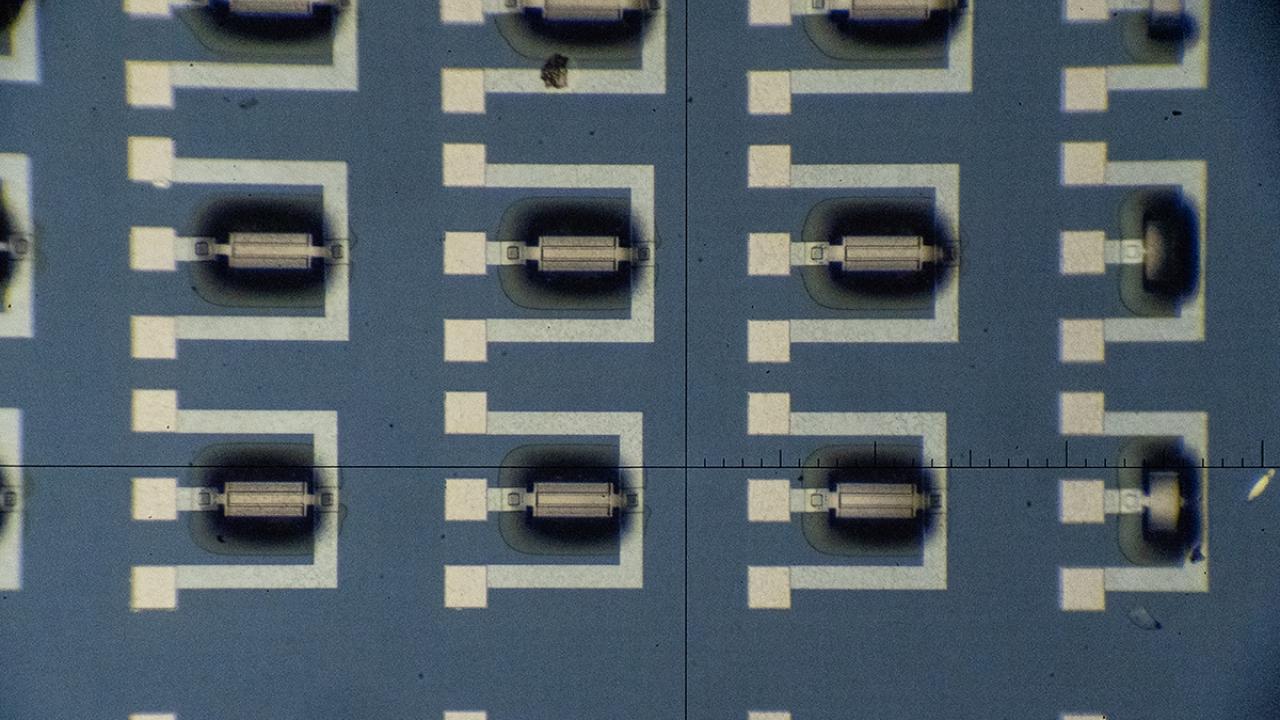
Over the past few years, Gomez-Diaz has developed the technology that serves as the basis of the project in the cleanroom at the Center for Nano-MicroManufacturing at UC Davis, where he builds hundreds of detectors within a single chip.
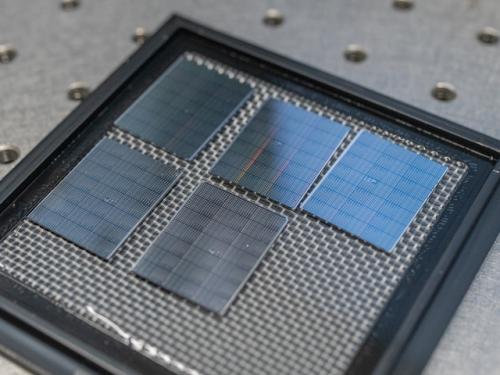
Chips sit on a table, visible to the naked eye. Gomez-Diaz and his team carefully arrange a neural network and hundreds of detectors decorated with metasurfaces onto each chip. (Steven Trinh/UC Davis)
The novel nanotechnology uses mechanical electromechanical systems with an ultrathin metasurface, or a two-dimensional plane of resonators featuring periodic arrangements.
The final product, the team explains, will be a small device that a patient can spit into, like a 23andMe genetic test. Once the device receives the sample, the patient can press a button to begin the diagnostic process, which the team expects to take no more than a few seconds.
"What we're trying to do is miniaturize these huge machines that we have in the lab into a small box that the average person can use," Gomez-Diaz said.
In a sense, the technology may move to democratize the screening process, putting the power of complicated machines into the hands of individuals.
"We believe this project is the early step towards a platform that can be miniaturized into a small wearable device that can monitor your cancer state non-invasively throughout the day," Carney said.
The original article written by Matt Marcure can be found here.